CONCEPT-DRIVEN APPROACH
FACE-Q Aesthetics is a rigorously developed patient-reported outcome measure that can be used to measure outcomes for any type of surgical or minimally invasive facial aesthetic treatment. FACE-Q Aesthetics was developed from concept elicitation interviews with 50 patients having surgical or minimally invasive treatments. To establish content validity, we conducted 35 cognitive debriefing interviews and obtained input from 26 experts. FACE-Q Aesthetics was then field-tested internationally. Adding to FACE-Q Aesthetics, we recently completed 26 new concept elicitation interviews. Data were used to create 3 scales measuring the important concept of natural. We also created item libraries for facial appearance and psychological wellbeing to provide an innovative means to customize short-form scales to maximize content validity.

MODULAR DESIGN
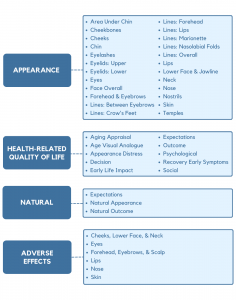
The conceptual framework for FACE-Q Aesthetics covers 4 domains: appearance, health-related quality of life, natural, and adverse effects. Each domain is composed of multiple independently functioning scales. The variety of scales provides flexibility to choose the subset of scales best suited to measure the outcomes of interest in any given study or clinical situation. An advantage of the modular structure, with individually scored scales, means it is possible to add new scales to fill any gap as these are identified. For example, for the concept of natural, we recently created a new module composed of 3 scales to provide a means to measure expectations and whether facial cosmetic treatments have achieved a natural result from the patient perspective.
Advancing Knowledge &
Improving Health Outcomes
INDUSTRY
Qualified by USA FDA as Medical Device Development Tool (MDDT) for use in research studies.
BENCHMARKING
Included in the UK Royal College of Surgeons national cosmetic surgery quality improvement initiative to measure outcomes for specific procedures.
PATIENT CARE
Used in clinical practice to measure outcomes from the patient perspective.
SCALE STRUCTURE
The FACE-Q module includes 37 independently functioning scales and 6 checklists that measure outcomes important to patients from their perspective. The FACE-Q modular approach allows clinicians and researchers to administer the subset of scales relevant to their situation.
In April 2022, 11 FACE-Q scales were qualified by the USA FDA as Medical Device Development Tools (MDDT). The qualified scales measure appearance (face, cheeks, lips, skin, nasolabial folds, and glabella lines), and health-related quality of life (aging appraisal, early life impact of treatment, satisfaction with outcome, psychological function, and social function).
FACIAL APPEARANCE
A comprehensive set of 24 scales measure appearance. Of this set, 17 scales measure appearance of the face overall and of specific facial features and areas, and 7 scales measure the appearance of facial lines overall and in specific areas of the face (e.g., nasolabial folds).
HEALTH-RELATED QUALITY OF LIFE
Two scales can be used to screen patients for unrealistic expectations and appearance-related distress. Age concerns are measured by an age appraisal scale and age visual analogue scale. Recovery from treatment is measured by 2 scales. The final 4 scales measure psychological and social function, satisfaction with treatment decision and outcome.
NATURAL
Three natural scales provide a means for patients to self-report their expectations before treatment as well as their outcomes after treatment. One outcome scale measures how natural the face looks after treatment (e.g., My facial features (eg, lips, cheeks) look balanced). The second scale measures what patients think about the results of their treatment (e.g., The treatment was not overdone).
ADVERSE EFFECTS
There are 6 checklists that can be used to measure adverse effects associated with aesthetic treatments. These checklists cover the cheeks, lower face and neck area, eyes, forehead, eyebrows and scalp area, lips, nose, and skin.
FACE-Q | AESTHETICS FRAMEWORK
CO-DEVELOPERS

Dr Anne Klassen

Dr Andrea Pusic