Research
Publications involving 1000s of women from numerous countries.
The BREAST-Q is a rigorously developed patient-reported outcome measure for use in cosmetic and reconstructive breast surgery and clinical practice. The BREAST-Q was developed from concept elicitation interviews with 48 women having different forms of breast surgery (augmentation, reduction and reconstruction). To establish content validity, we conducted focus groups and pilot testing with 58 women, and final cognitive debriefing interviews with 30 women. We also obtained input from clinical experts. The BREAST-Q was then field-tested in a sample of 1950 women (464 reduction). A further psychometric validation study was performed in an independent sample of 817 women (301 reduction). Since its publication in 2009, the BREAST-Q has been licensed for use by more than 4000 clinicians and researchers in >75 countries.
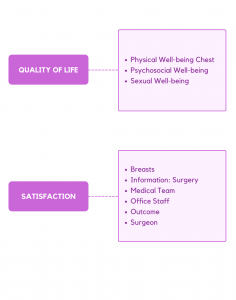
The BREAST-Q conceptual framework covers 2 domains: quality of life, and patient satisfaction. We developed independent modules for reduction/mastopexy, augmentation, and breast cancer (mastectomy, breast-conserving therapy, reconstruction). Each module is composed of multiple independently functioning scales. The variety of scales provides flexibility to choose the subset of scales best suited to measure the outcomes of interest in any given study or clinical situation. The modular structure, with individually scored scales, offers an advantage of adding new scales to fill any gap as these are identified. To this end, our team recently performed 58 new concept elicitation interviews and developed new scales for the cancer module, and a utility module for use in health economic studies. We are also developing a breast implant illness module.
Publications involving 1000s of women from numerous countries.
Included in international clinical trials and post-market surveillance studies.
Normative data based on 1000s of women.
Designed using a modern psychometric approach to facilitate use in patient care.
The BREAST-Q module for women who undergo breast reduction/mastopexy includes 9 independently functioning scales that measure outcomes and experience of health care from the patient perspective. Clinicians and researchers are able to administer the subset of scales relevant to their situation.
Measures psychosocial well-being with items that ask about body image and a woman’s confidence in social settings.
Measures pain, rashes, and difficulty with physical activities, such as lifting, running or exercising.
Asks about feelings of sexual attractiveness (clothed and unclothed), sexual confidence, and comfort level during sex.
Items ask about breast size, how bras fit, symmetry, and how the breasts look when clothed or unclothed.
Four scales measure patients’ experience of healthcare. The first scale measures satisfaction with information and the other 3 scales measure satisfaction with members of the healthcare team, i.e., surgeon, medical team, and office staff.
Measures the outcome of surgery and whether or not expectations were met.