RESEARCH
Can be used in research studies and clinical trials to study different approaches to treatments.
FACE-Q Head & Neck Cancer is a rigorously developed patient-reported outcome measure that can be used to measure outcomes for any type of head and neck cancer surgery. This FACE-Q module was developed from concept elicitation interviews with 26 patients with a range of different types of cancer. FACE-Q Head & Neck Cancer was then field-tested in a USA study involving 219 patients.

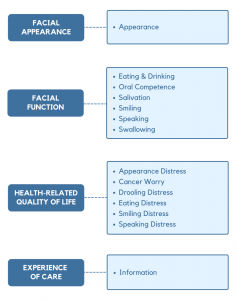
The conceptual framework for FACE-Q Head & Neck Cancer covers 4 domains: appearance, function, quality of life, and experience of care. Each domain is composed of 1 or more independently functioning scales. The variety of scales provides flexibility to choose the subset of scales best suited to measure the outcomes of interest in any given study or clinical situation.
Can be used in research studies and clinical trials to study different approaches to treatments.
Carefully designed to meet the requirements of regulatory bodies.
Used to benchmark outcomes in quality improvement initiatives.
Increasingly used in clinical practice to measure outcomes from the patient perspective.
The FACE-Q Head & Neck Cancer includes 14 independently functioning scales that measure outcomes important to patients from their perspective. Clinicians and researchers are able to administer the subset of scales relevant to their situation.
A single scale measures the appearance of the face overall, e.g., looks unattractive, disfigured, uneven).
Six scales measure the following facial functions: eating/drinking, oral competence, salivation, smiling, speaking and swallowing.
Six scales measure aspects of quality of life, including 4 scales that ask about distress in relation to facial functions (eating, drooling, smiling, and speaking). The final 2 scales measure appearance distress and cancer worry.
A single scale measures satisfaction with information provided about issues such as complications, healing and recovery time, and details of the procedure.