Research
Can be used in research studies and clinical trials to study different surgical approaches.
The EAR-Q was developed from concept elicitation interviews with 25 participants: 14 with microtia, 9 with prominent ears, and 2 with another ear condition. The scales were refined with patient and expert input and translated into multiple languages. The EAR-Q was then field-tested in an international sample of 863 participants: 607 with microtia, 145 with prominent ears, and 111 with another ear condition.

The EAR-Q was developed and tested in children and young adults aged 8 to 29 years with any type of ear condition. The EAR-Q includes 6 independently functioning scales and 3 single item questions. The variety of scales provides flexibility to choose the subset of scales best suited to measure the outcomes of interest in any given study or clinical situation.
Can be used in research studies and clinical trials to study different surgical approaches.
Carefully designed to meet the requirements of regulatory bodies.
Can be used to benchmark outcomes in quality improvement initiatives.
Designed using a modern psychometric approach to facilitate use in patient care.
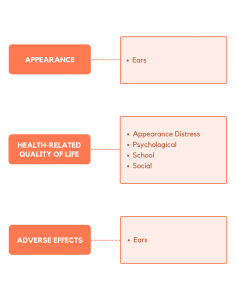
The EAR-Q is a rigorously designed patient-reported outcome measure that can be used to collect and compare evidence-based outcomes data from patients with any kind of ear condition. The EAR-Q measures 3 overarching domains. Each domain is composed of 1 or more independently functioning scales. Clinicians and researchers are able to administer the subset of scales relevant to their situation.
The EAR-Q includes a 10-item appearance scale. The items ask how the ear(s) look from the patient perspective (e.g., shape and size, look in photos, from the side and when wearing a hat). There are also 3 single items that ask about how ear scars look and feel and how hearing aids look.
The EAR-Q includes 4 scales that measure aspects of health-related quality of life, including psychological, social and school function and appearance-related distress.
The EAR-Q includes a 10-item adverse effects scale. This scale asked patients to rate the severity of a range of adverse effects (e.g., itchy, painful, numb, bruised, tingly).